Seasonal flu vaccines available in Europe come in multiple flavours: they protect against 3 or 4 virus strains, they can be inactivated or live attenuated, and may or may not contain an adjuvant… on top of this, several brands of each flavour may exist. DRIVE aims to estimate vaccine effectiveness for the flu vaccine brands used in Europe.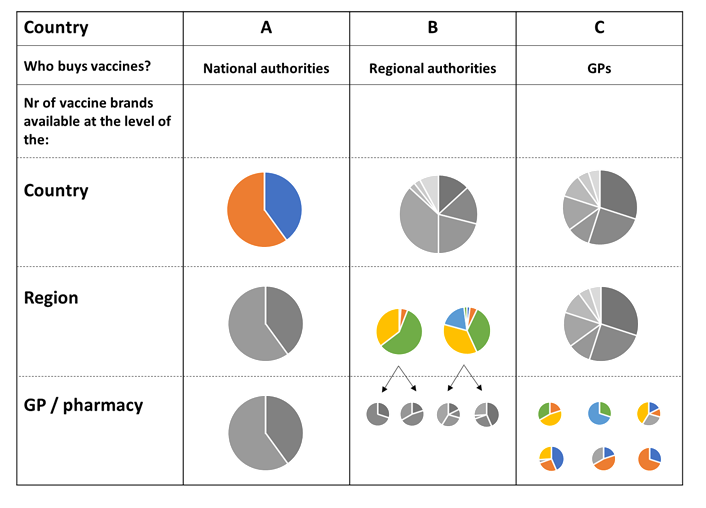
To this end, we would like to know: which vaccine brands are used where? What might be seen as quite a simple question, surprisingly doesn’t have an easily accessible answer.
We started by looking at vaccine procurement systems in 14 European countries.
How do the vaccines arrive from the manufactures to the population – are they bought by health authorities or does the local general practitioner (GP) or pharmacist decide which vaccines to buy? How does this affect the number of brands that will be available in a population? Is it possible to know well before the yearly flu shot campaigns start which vaccines will be used where?
To answer these questions we conducted interviews with experts on flu vaccine procurement from public health institutions and manufacturers.
Finally, we want to know: can knowledge on these processes help DRIVE to determine where to set up studies so that vaccine effectiveness can be obtained for all the different vaccine brands? Come to find out at ESCAIDE!
Anke Stuurman
Presentation at ESCAIDE be Anke Stuurman on the 23.11.: Seasonal influenza vaccine procurement systems in Europe
Moderated poster session
Day 3: 15:40-16:40
Track 23: Policy approaches and evaluation C23.6.
Related:
EAN Prize winners from ESCAIDE 2017 – Best poster presentation:
Ulrike Baum, “Interactive vaccination coverage maps – a multipurpose tool for programme monitoring and health system development”
Ulrike is currently a member of DRIVE consortium and will be giving this year presentations on Finnish register-based cohort studies in influenza vaccine effectiveness.
