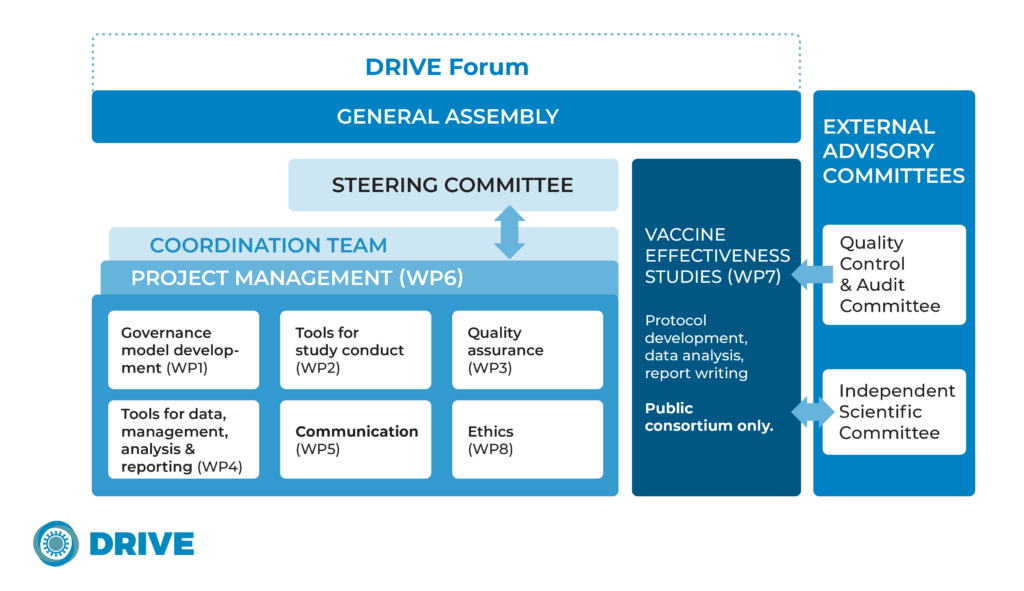
Project Structure
DRIVE is divided into eight Work Packages (WP), with seven co-chaired by a public and a private partner. The co-chairs jointly contribute to the tasks and deliverables assigned to the WP.
The WP responsible for the pilot studies is led by public institutions only; vaccine manufacturers do not participate in the execution of studies and do not have access to the raw data.
FISABIO is the DRIVE Coordinator, acting as the central point of contact between the consortium and IMI. Sanofi Pasteur is the Project Leader, coordinating the participation of vaccine manufacturers and also supporting the project management tasks. Both FISABIO and Sanofi Pasteur are supported by the Project Manager, Synapse.
The WP leaders and co-ordinators form the DRIVE Steering Committee, which is responsible for managing the deliverables of the project and making decisions as required. There are equal voting rights between all partners.
To guarantee the scientific independence of the studies, the role of each partner is clearly defined and traceable. Vaccine manufacturers will provide written comments on the scientific deliverables to an Independent Scientific Committee who will evaluate and endorse the studies’ deliverables.
A Quality Control & Audit Committee advises on the compliance and quality of the studies.
All DRIVE partners together constitute a General Assembly that convenes annually.