More than 100 influenza and COVID-19 experts came together for DRIVE’s 4th Annual Meeting, which took place online on November the 30th.
The meeting kicked off with a short introduction from DRIVE’s project Coordinator, Prof Javier Díez-Domingo, who emphasized the “unprecedented level of multi-stakeholder collaboration” and the contributions of both DRIVE and COVIDRIVE projects to this landscape.

Next up were Anke Stuurman (P95) & Laurence Pagnon (Sanofi Pasteur) who introduced the project’s achievements and the challenges encountered during the first 4 years of DRIVE. “In 2017/18 influenza activity was the highest we had seen in DRIVE. The start of the COVID-19 pandemic had a large effect on influenza activity and in 2020/21 influenza rates were very low”, they commented.
Their presentation also included a feasibility evaluation of brand-specific influenza vaccine effectiveness (VE) in a Public Private Partnership (PPP) framework, as well as results from four years of work.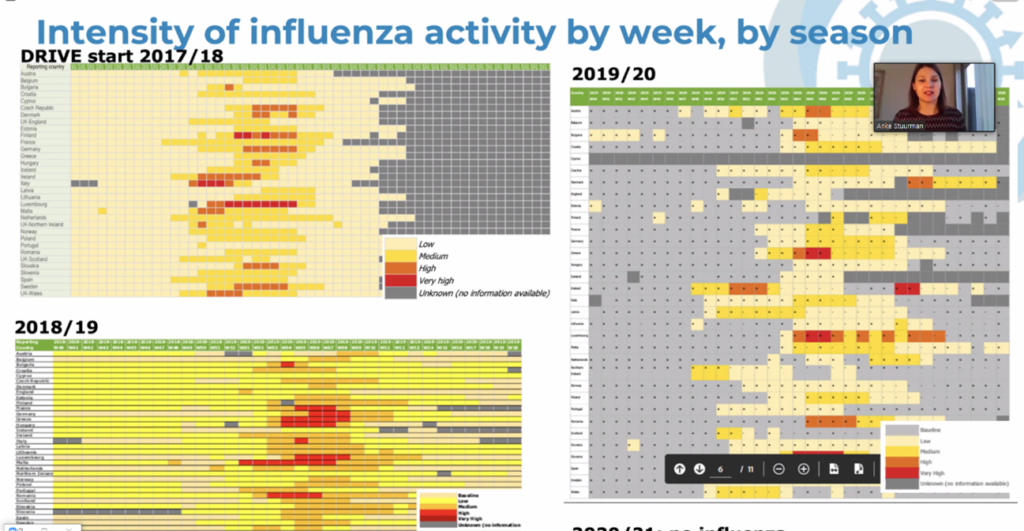
Manuela Mura, a representative of the European Medicines Agency, presented next on the joint platform created by the ECDC and EMA for monitoring vaccine effectiveness. “What is important for EMA is that robust data is generated in real time. We need to make sure that everyone’s expertise is used in the best possible way”, Manuela said. She also provided an update on the current status of COVID-19 and influenza and on the plans for the coming 2021-22 season.
Next on the agenda, the COVIDRIVE consortium was officially presented to DRIVE partners and external stakeholders. Kaat Bollaerts (P95) and Toni Carmona (FISABIO), COVIDRIVE co-coordinators, introduced the genesis of the partnership, the framework, and the first COVID-19 vaccine effectiveness study performed.
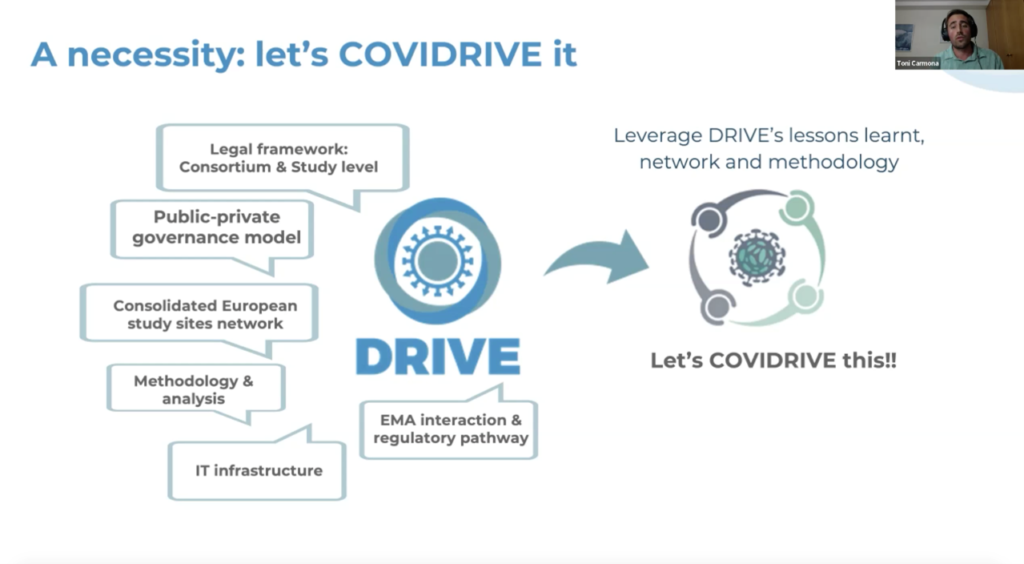
Lastly, the attendees learned from the FDA’s experience in investigating vaccine effectiveness using real world evidence thanks to Dr. Héctor Izurieta, senior epidemiologist at the office of biostatistics and Epidemiology in the Center for Biologics Evaluation and Research of the Food and Drug Administration and member of DRIVE’s Independent Scientific Committee.
 The session was closed by Cédric Mahé (Sanofi Pasteur), DRIVE EFPIA lead, and was an opportunity to discuss the new European landscape with stakeholders. Questions such as if there is a plan to broaden the DRIVE network outside the EU or strategies on how the PPP model can be replicated on a global scale were topics of animated discussion.
The session was closed by Cédric Mahé (Sanofi Pasteur), DRIVE EFPIA lead, and was an opportunity to discuss the new European landscape with stakeholders. Questions such as if there is a plan to broaden the DRIVE network outside the EU or strategies on how the PPP model can be replicated on a global scale were topics of animated discussion.

The DRIVE project will come to an end in June 2022 but we can confirm that, despite the pandemic’s direct impact on the project results and activities, DRIVE partners, study sites and collaborators have been able to accomplish so much together. Looking forward to meeting up again for the last DRIVE Annual Forum (hopefully face to face!) in 2022.
