Study findings will help to inform decision-making by public health institutes, vaccine companies and regulatory authorities in Europe
19/07/2021 – A new public-private partnership – COVIDRIVE – announced today that it will begin studies to assess the effectiveness of multiple COVID-19 vaccines in Europe to support the region’s public health response and to address the vaccine companies’ regulatory obligations. This multi-stakeholder partnership brings together public institutions, small medium enterprises and vaccine companies, including FISABIO (Spain), P95 (Belgium), THL (Finland), AstraZeneca (UK), CureVac (Germany), Janssen (Belgium), Sanofi-Pasteur (France) and GSK (Belgium).
The partnership will conduct several studies to analyse COVID-19 vaccine effectiveness in real-world conditions to complement what is known from robust clinical trials conducted for marketing authorisations. In addition to overall effectiveness for each brand of vaccine, key areas of interest include: duration of vaccine protection, effectiveness against disease caused by newly emerging SARS-CoV-2 strains, effectiveness against severe COVID-19 disease and effectiveness in special risk groups such as immunocompromised, frail individuals or subjects with chronic conditions or existing comorbidities.
COVIDRIVE will leverage an existing influenza vaccine effectiveness platform (DRIVE), which has provided annual brand-specific influenza vaccine effectiveness estimates to the European Medicines Agency (EMA) since 2017.
AstraZeneca and Janssen will be the first pharmaceutical companies in the partnership to launch their brand-specific COVID-19 vaccine effectiveness assessments through COVIDRIVE, starting in July 2021.
Thomas Verstraeten, P95’s CEO and co-coordinator of COVIDRIVE, explains: “COVIDRIVE leverages the work generated during DRIVE’s four years of life. The current COVID-19 pandemic highlights the need for a public-private collaborative environment to generate vaccine effectiveness data to advise the design of national immunisation programmes and to fulfil the effectiveness requirements established by the regulatory authorities”.
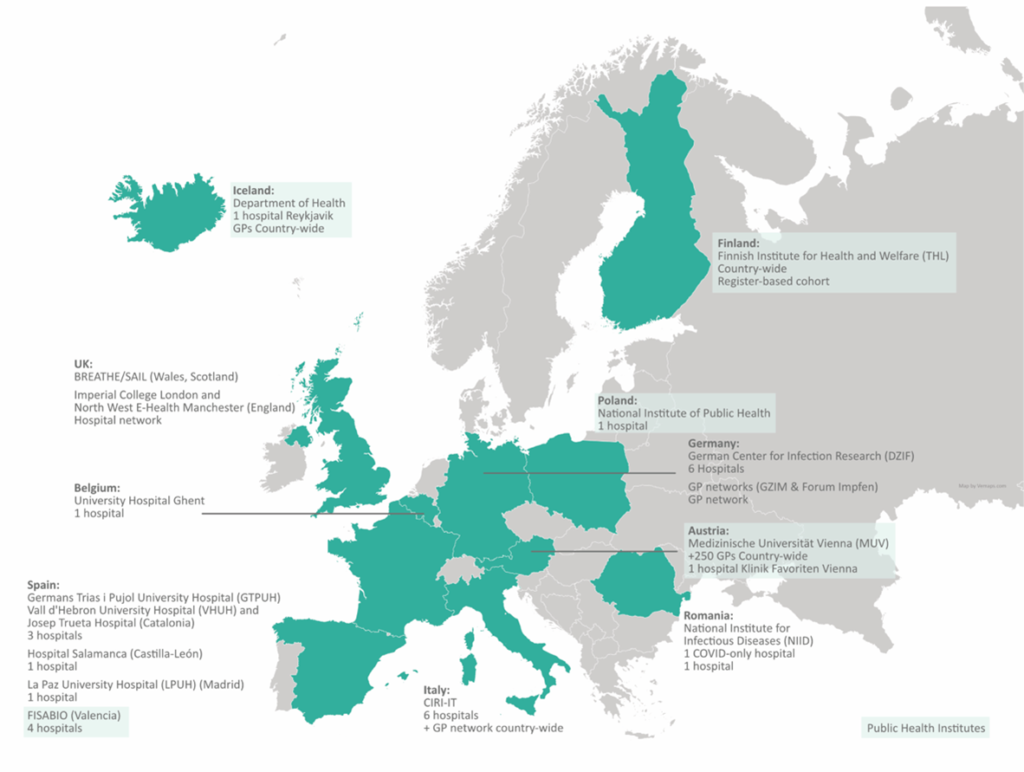
COVIDRIVE has developed an extensive COVID-19 observational study network in which more than 40 hospitals and more than 500 General Practitioners in 10 European countries have expressed interest in participating, including Austria, Belgium, Finland, Germany, Iceland, Italy, Poland, Romania, Spain and the United Kingdom.
Prof Javier Díez-Domingo, Head of the Vaccines Research department of the FISABIO foundation, and COVIDRIVE co-coordinator comments: “We will be prioritizing the study of the effectiveness of the COVID-19 vaccines against COVID-19 related hospitalisations. The rationale behind this prioritization is that COVID-19 hospitalisations are one of the main reasons for national and regional governments to impose public health measures such as shutdowns, social distance and wearing of masks to relieve the disease burden on the healthcare systems. Producing accurate and timely information on how well the different COVID-19 vaccines protect against hospitalisations and remain protective over time is essential to successfully manage the pandemic”.
Dr Su-Peing Ng, Chief Medical Officer of Sanofi Pasteur commented “Through public and private scientific partnership, COVIDRIVE will deliver essential data and insights to support COVID-19 control and advance disease understanding. We are confident that the anticipated studies will provide important insights into the value brought by vaccination against COVID-19”.
For more details on COVIDRIVE please check our website https://covidrive.eu
The master protocol developed by COVIDRIVE will be soon available at the EU PAS register of ENCePP and in the COVIDRIVE website.
If you would like to request a COVID-19 vaccine effectiveness study and/or participate as a partner/study site in COVIDRIVE, send an email to COVIDRIVE Coordination team (info@covidrive.eu)