 Seasonal influenza (flu) is an infectious disease with a major impact on European citizens because of its high incidence rate and severity. Although vaccination is the best way to protect against influenza, several factors affect how well vaccines will work in a given influenza season. Europe-wide research is needed to understand how different influenza vaccines perform each influenza season.
Seasonal influenza (flu) is an infectious disease with a major impact on European citizens because of its high incidence rate and severity. Although vaccination is the best way to protect against influenza, several factors affect how well vaccines will work in a given influenza season. Europe-wide research is needed to understand how different influenza vaccines perform each influenza season.
The DRIVE consortium has built a research platform for studying brand-specific influenza vaccine performance, known as “vaccine effectiveness” during its pilot years 2017–2018. As more study sites join the DRIVE network, DRIVE can expect to answer the regulatory and public health need of better understanding influenza vaccine effectiveness.
Why EU-wide research on brand-specific influenza vaccine effectiveness is needed
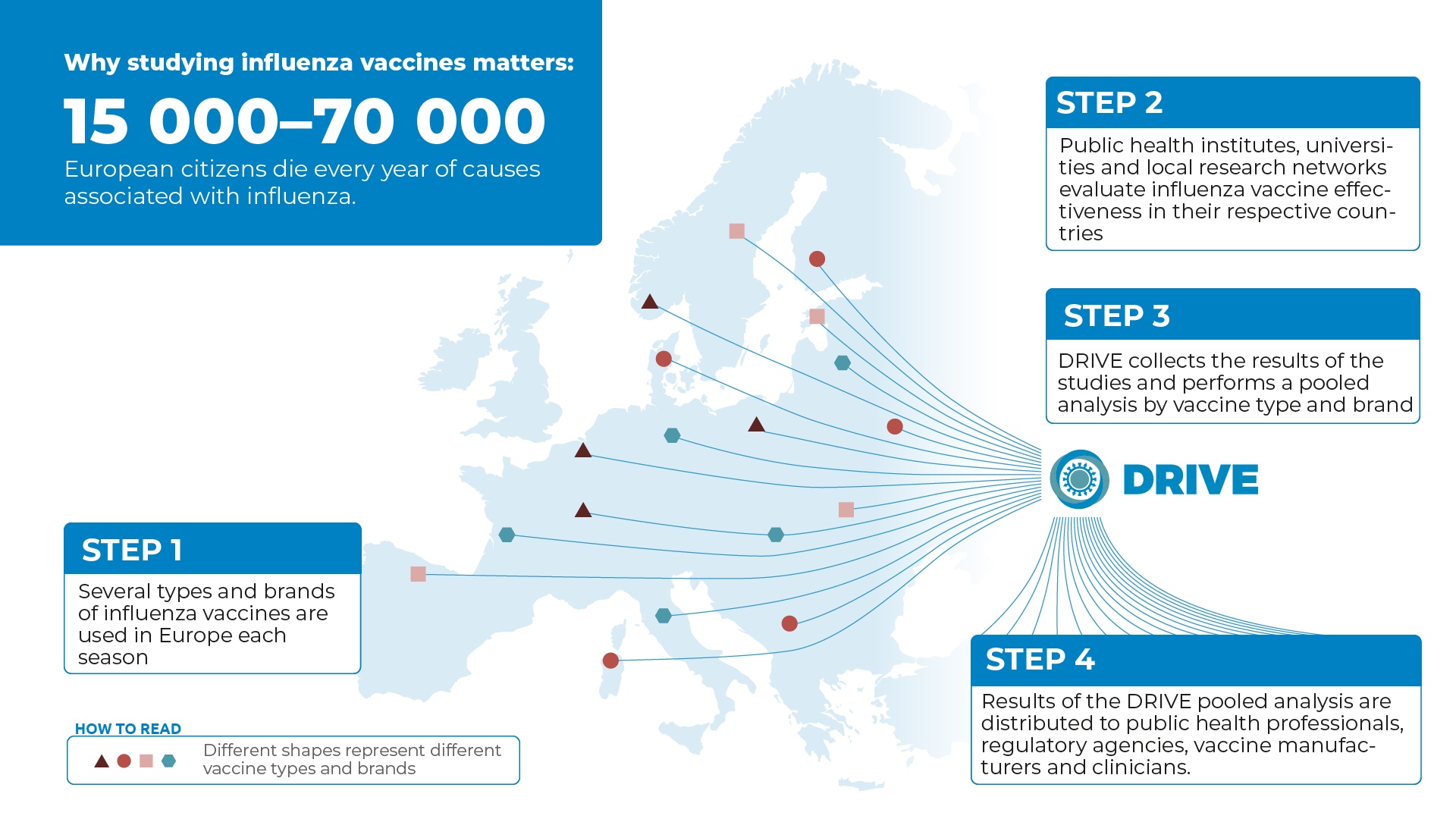
Several types and brands of influenza vaccines are used in Europe each season. Some vaccines contain inactivated viruses or parts of viruses and are given as injections. Another type of vaccine contains live but weakened viruses and is given as nasal spray. Influenza vaccines are also made using different manufacturing processes. Some are produced in eggs, others in cells. Some vaccines contain adjuvants that aim to promote a better immune response.
Vaccine type and brand availability differs by country, but also by region or even at the clinic level and this may change each season, depending on by whom, how and when influenza vaccines are procured.
Influenza viruses are continuously changing. The World Health Organization conducts year-round surveillance of flu viruses globally and recommends the strains to be included in influenza vaccines each season. The aim is to protect people against the viruses anticipated to circulate predominately in the upcoming season.
If a change in the structure of an influenza virus is detected after the vaccine production has started – either in the circulating viruses or in the vaccine virus during production – there is no time to change the vaccine composition. This can result in a so-called mismatch which lowers the effectiveness of the vaccine – as may have happened during the 2017/18 season. Considering the complexity of accurately evaluating the performance of each brand of influenza vaccine each year, a large amount of data is needed in a real-world setting.
How does drive study brand-specific influenza vaccine effectiveness?
Influenza vaccine effectiveness studies assess how well flu vaccines perform by comparing the incidence of the disease in those who have been vaccinated against those who have not received the vaccine.
European public health institutes, universities, and local research networks evaluate influenza vaccine effectiveness on a yearly basis. The studies are often conducted in primary care or among hospitalized patients and some make use of large databases that are gathered during routine healthcare practices.
However, in most European countries only a few different vaccine brands are used, or different brands are available but the use of each may be limited. This means the data from one or even a few countries is not enough to gain extensive insight on brand-specific effectiveness.
Therefore, DRIVE collects the results of independently conducted studies from several European countries and analyses them together to reach as broad a coverage of vaccine brands as possible.
As understanding the effectiveness of vaccines is a priority for both public health officials and vaccine manufacturers, DRIVE operates as a public-private partnership. The studies themselves are conducted by public partners without the involvement of vaccine manufacturers and are reviewed by an independent scientific committee. The DRIVE consortium also includes one non-government organization to represent the patients’ voice.
In order to make sure that relevant information regarding influenza vaccine performance is available to European citizens, the European Medicines Agency (EMA) has issued a guideline that requests effectiveness evaluation for all brands used in the EU. Results of the DRIVE analysis are distributed also to public health professionals, regulatory agencies, vaccine manufacturers and clinicians.
Why studying influenza vaccine matters
According to the European Centre for Disease Prevention and Control (ECDC), seasonal influenza causes 4–50 million symptomatic cases in Europe each year, and 15 000–70 000 European citizens die every year of causes associated with influenza. The yearly economic and healthcare burden of influenza is substantial.
Download the summary as a PDF
Read more
The scientific summary
The full report
The consortium members
Follow @drive_eu at Twitter and LinkedIn – and join the discussion with the hashtag #DRIVEflu.
The DRIVE project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 777363. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
http://www.imi.europa.eu
